Close
Important Safety Information for Parsabiv®
Contraindication: Parsabiv® (etelcalcetide) is
contraindicated in patients with known hypersensitivity to etelcalcetide or any of its excipients.
Hypersensitivity reactions, including face edema and anaphylactic reaction, have occurred.
Hypocalcemia: Parsabiv® lowers serum calcium and can
lead to hypocalcemia, sometimes severe. Significant lowering of serum calcium can cause QT interval
prolongation and ventricular arrhythmia. Patients with conditions that predispose to QT interval
prolongation and ventricular arrhythmia may be at increased risk for QT interval prolongation and
ventricular arrhythmias if they develop hypocalcemia due to Parsabiv®. Closely monitor
corrected serum calcium and QT interval in patients at risk on Parsabiv®.
Significant reductions in corrected serum calcium may lower the threshold for seizures. Patients with a
history of seizure disorder may be at increased risk for seizures if they develop hypocalcemia due to
Parsabiv®. Monitor corrected serum calcium in patients with seizure disorders on
Parsabiv®.
Concurrent administration of Parsabiv® with another oral calcimimetic could result in
severe, life-threatening hypocalcemia. Patients switching from cinacalcet to Parsabiv®
should discontinue cinacalcet for at least 7 days prior to initiating Parsabiv®. Closely
monitor corrected serum calcium in patients receiving Parsabiv® and concomitant therapies
known to lower serum calcium.
Measure corrected serum calcium prior to initiation of Parsabiv®. Do not initiate in
patients if the corrected serum
calcium is less than the lower limit of normal. Monitor corrected serum calcium within 1 week after
initiation or dose
adjustment and every 4 weeks during treatment with Parsabiv®. Measure PTH 4 weeks after
initiation or dose adjustment of
Parsabiv®. Once the maintenance dose has been established, measure PTH per clinical
practice.
Worsening Heart Failure: In Parsabiv
® clinical studies,
cases of hypotension, congestive heart failure, and decreased myocardial performance have been reported.
Closely monitor patients treated with Parsabiv
® for worsening signs and symptoms of heart
failure.
Upper Gastrointestinal Bleeding: In clinical studies, 2 patients
treated with Parsabiv® in 1253 patient years of exposure had upper gastrointestinal (GI)
bleeding at the time of death. The exact cause of GI bleeding in these patients is unknown and there
were too few cases to determine whether these cases were related to Parsabiv®.
Patients with risk factors for upper GI bleeding, such as known gastritis, esophagitis, ulcers or severe
vomiting, may be at increased risk for GI bleeding with Parsabiv®. Monitor patients for
worsening of common Parsabiv® GI adverse reactions and for signs and symptoms of GI
bleeding and ulcerations during Parsabiv® therapy.
Adynamic Bone: Adynamic bone may develop if PTH levels are chronically
suppressed.
Adverse Reactions: In clinical trials of patients with secondary HPT
comparing Parsabiv® to placebo, the most common adverse reactions were blood calcium
decreased (64% vs. 10%), muscle spasms (12% vs. 7%), diarrhea (11% vs. 9%), nausea (11% vs. 6%),
vomiting (9% vs. 5%), headache (8% vs. 6%), hypocalcemia (7% vs. 0.2%), and paresthesia (6% vs. 1%).
Indication
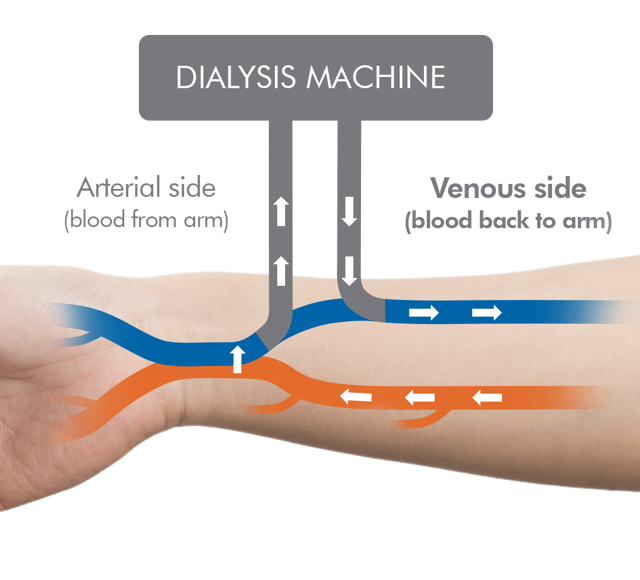
Parsabiv® (etelcalcetide) is indicated for the treatment of secondary hyperparathyroidism
(HPT) in adult patients with chronic kidney disease (CKD) on hemodialysis.
Limitations of Use: Parsabiv® has not been studied in adult patients with parathyroid carcinoma, primary
hyperparathyroidism, or with CKD who are not on hemodialysis and is not recommended for use in these
populations.
Limitations of Use:
Parsabiv® has not been studied in adult patients with parathyroid carcinoma, primary
hyperparathyroidism, or with CKD who are not on hemodialysis and is not recommended for use in these
populations.
Please see Parsabiv® (etelcalcetide) full Prescribing
Information.