Contraindication: Parsabiv® (etelcalcetide) is contraindicated in patients with known hypersensitivity to
etelcalcetide or any of its excipients. Hypersensitivity reactions, including face edema and anaphylactic reaction,
have occurred.
Hypocalcemia: Parsabiv® lowers serum calcium and can lead to hypocalcemia, sometimes severe. Significant
lowering of serum calcium can cause QT interval prolongation and ventricular arrhythmia. Patients with
conditions that predispose to QT interval prolongation and ventricular arrhythmia may be at increased risk for
QT interval prolongation and ventricular arrhythmias if they develop hypocalcemia due to Parsabiv®. Closely
monitor corrected serum calcium and QT interval in patients at risk on Parsabiv®.
Significant reductions in corrected serum calcium may lower the threshold for seizures. Patients with a history of
seizure disorder may be at increased risk for seizures if they develop hypocalcemia due to Parsabiv®. Monitor
corrected serum calcium in patients with seizure disorders on Parsabiv®.
Concurrent administration of Parsabiv® with another oral calcimimetic could result in severe, life-threatening
hypocalcemia. Patients switching from cinacalcet to Parsabiv® should discontinue cinacalcet for at least 7 days
prior to initiating Parsabiv®. Closely monitor corrected serum calcium in patients receiving Parsabiv® and
concomitant therapies known to lower serum calcium.
Measure corrected serum calcium prior to initiation of Parsabiv®. Do not initiate in patients if the corrected serum
calcium is less than the lower limit of normal. Monitor corrected serum calcium within 1 week after initiation or
dose adjustment and every 4 weeks during treatment with Parsabiv®. Measure PTH 4 weeks after initiation or
dose adjustment of Parsabiv®. Once the maintenance dose has been established, measure PTH per clinical
practice.
Worsening Heart Failure: In Parsabiv® clinical studies, cases of hypotension, congestive heart failure, and
decreased myocardial performance have been reported. Closely monitor patients treated with Parsabiv® for
worsening signs and symptoms of heart failure.
Upper Gastrointestinal Bleeding: In clinical studies, 2 patients treated with Parsabiv® in 1253 patient years of
exposure had upper gastrointestinal (GI) bleeding at the time of death. The exact cause of GI bleeding in these
patients is unknown and there were too few cases to determine whether these cases were related to Parsabiv®.
Patients with risk factors for upper GI bleeding, such as known gastritis, esophagitis, ulcers or severe vomiting,
may be at increased risk for GI bleeding with Parsabiv®. Monitor patients for worsening of common Parsabiv® GI
adverse reactions and for signs and symptoms of GI bleeding and ulcerations during Parsabiv® therapy.
Adynamic Bone: Adynamic bone may develop if PTH levels are chronically suppressed.
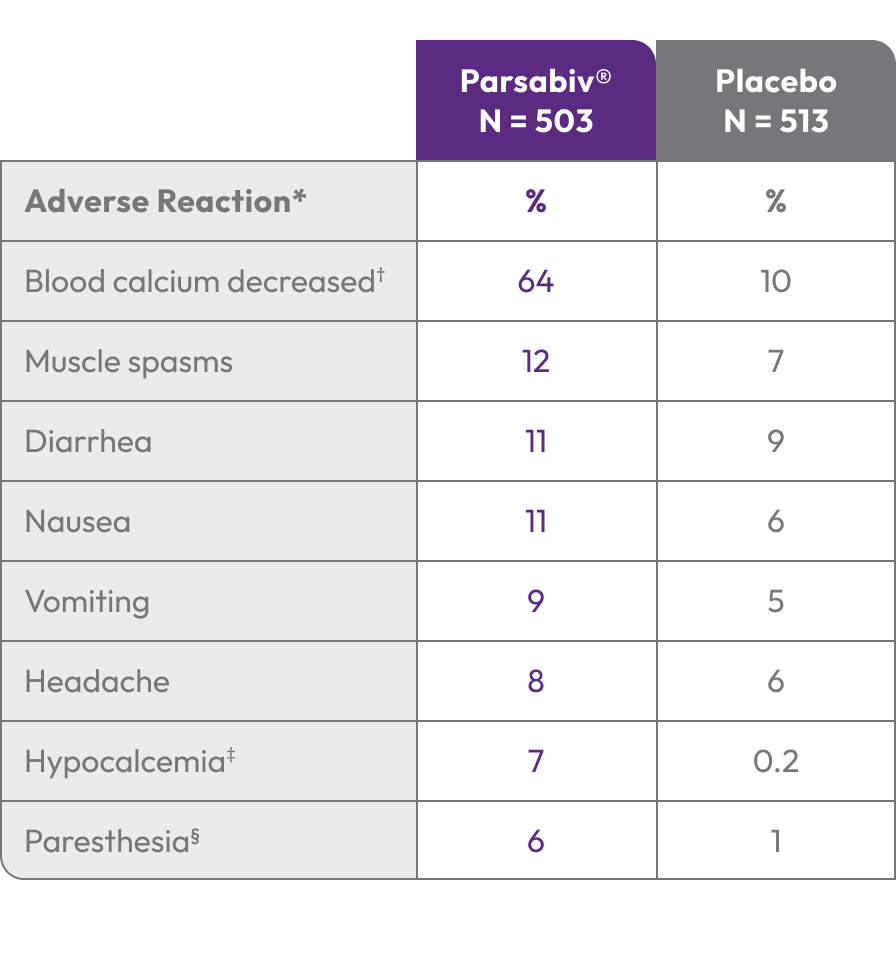
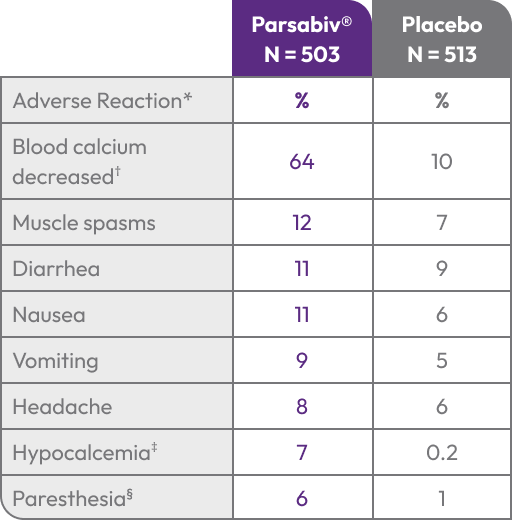
Adverse Reactions: In clinical trials of patients with secondary HPT comparing Parsabiv® to placebo, the most
common adverse reactions were blood calcium decreased (64% vs. 10%), muscle spasms (12% vs. 7%), diarrhea
(11% vs. 9%), nausea (11% vs. 6%), vomiting (9% vs. 5%), headache (8% vs. 6%), hypocalcemia (7% vs. 0.2%), and
paresthesia (6% vs. 1%).
Indication
Parsabiv® (etelcalcetide) is indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients
with chronic kidney disease (CKD) on hemodialysis.
Limitations of Use: Parsabiv® has not been studied in adult patients with parathyroid carcinoma, primary
hyperparathyroidism, or with CKD who are not on hemodialysis and is not recommended for use in these
populations.
Please see Parsabiv® (etelcalcetide) full Prescribing Information.