

Life is not a
controlled study.
Real-world evidence matters.
-
Placebo-controlled studies
-
Real-world evidence
-
Head-to-head study
Parsabiv® (etelcalcetide) safety and efficacy were evaluated
in 2
placebo-controlled
studies1, 3-7
Study Design


-
*Stratified by region, mean screening iPTH, and recent oral cinacalcet use.
- Two phase 3, 26-week, multicenter, randomized, double-blind, placebo-controlled clinical studies comparing Parsabiv® (etelcalcetide) with placebo in patients with CKD on hemodialysis with iPTH > 400 pg/mL and corrected calcium ≥ 8.3 mg/dL; N = 1023
- Parsabiv® or placebo administered TIW into the venous line of the dialysis circuit at the end of hemodialysis during rinse back or intravenously after rinse back
- Patients in both treatment arms could be treated with vitamin D sterols and/or phosphate binders, if prescribed
- Mean baseline iPTH in the Parsabiv® group and placebo group were 847 pg/mL and 836 pg/mL, respectively
- During the EAP, the average weekly dose of active vitamin D (IV paricalcitol equivalent) was 16.7 µg in the Parsabiv® group and 14.5 µg in the placebo group
- The average dose of Parsabiv® at the time of the EAP (defined as weeks 20 through 27, inclusive) was 7.2 mg TIW
Titration
- The starting dose of Parsabiv® was 5 mg TIW at the end of hemodialysis
- The dose was titrated at weeks 5, 9, 13, and 17 to achieve predialysis serum iPTH ≤ 300 pg/mL. The dose could be increased in 2.5 mg or 5 mg increments based on predialysis iPTH and cCa concentrations
- The minimum dose was 2.5 mg and the maximum dose was 15 mg
- Parsabiv® was withheld if any of the following were observed: iPTH < 100 pg/mL (two consecutive measurements), corrected calcium < 7.5 mg/dL, symptomatic hypocalcemia, other ongoing adverse events
- Investigators were blinded to central laboratory serum PTH values, and routine local PTH monitoring during the study was suspended
78% of patients who received Parsabiv®
achieved > 30% reduction in mean PTH8
Percentage of patients achieving > 30% reduction in mean iPTH from baseline


One-third of patients given Parsabiv® had a reduction in PTH by week 4*—and by week 8 that number nearly doubled9
Post-hoc analysis: Time to first occurrence* of > 30% reduction in iPTH from combined placebo-controlled studies



Parsabiv® + vitamin D and/or phosphate binders†;
n = 509

Placebo + vitamin D and/or phosphate binders†;
n = 514
- *Timepoint when > 30% reduction in iPTH was first observed.
- †Vitamin D and/or phosphate binders, if prescribed.3
Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
Rolling averages of 3 iPTH values (from previous, current, and next visit) were used.
- The starting dose of Parsabiv® (etelcalcetide) was 5 mg TIW at the end of hemodialysis
- The dose was titrated at weeks 5, 9, 13, and 17 to achieve predialysis serum iPTH ≤ 300 pg/mL. The dose could be increased in 2.5 mg or 5 mg increments based on predialysis iPTH and cCa concentrations
Parsabiv® provided significant reductions across 3 key
sHPT lab values vs placebo8,10,*
Mean iPTH, phosphate, and corrected calcium over time
SCROLL OVER TO VIEW FULL CHART







Parsabiv® + vitamin D and/or phosphate binders§

Placebo + vitamin D and/or phosphate binders§

Switched from placebo in parent studies to Parsabiv® + vitamin D and/or phosphate binders§
- *P < 0.001 (mean percent change in EAP, defined as weeks 20 through 27).
- †Values represent mean iPTH during EAP, defined as weeks 20 through 27, inclusive.7
- ‡Value represents iPTH measured at the first hemodialysis session in week 79.9
- §Vitamin D and/or phosphate binders, if prescribed.3
Data pooled across two placebo-controlled parent studies and a subsequent open-label extension (OLE) study, starting from the baseline of the parent study until the end or the prespecified cutoff date of the OLE study, whichever was earlier. Weeks 27 to 31 were the 30-day drug-free period (the 30-day follow-up period of the phase 3 study before entry into the extension study).4 During the OLE, the starting dose of Parsabiv® for all subjects was 5 mg. The Parsabiv® (etelcalcetide) dose could be increased at OLE weeks 5, 9, 17, 25, 33, 41, and 49 to a maximum dose of 15 mg to achieve predialysis serum iPTH ≤ 300 pg/mL while maintaining appropriate serum cCa concentrations. Investigators were blinded to iPTH results during the first 10 weeks of treatment. Subsequent dose adjustment was determined by the investigator per protocol guidelines. The average weekly dose of Parsabiv® was 21.3 mg and 20.0 mg at 6 months and 12 months, respectively.11
Mean % (absolute) change from baseline



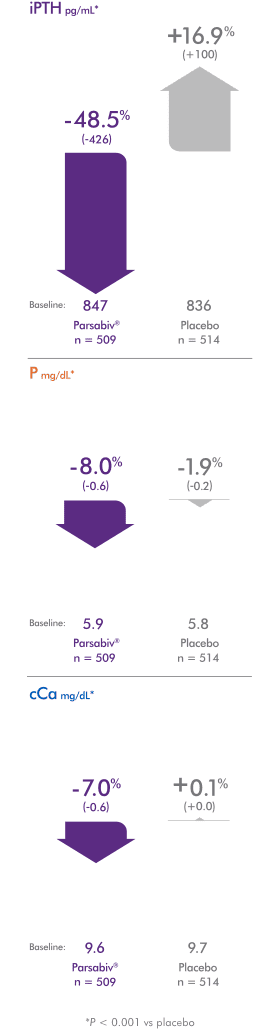

*P < 0.001 vs placebo
The majority of patients given Parsabiv® achieved the KDIGO®
goal range* for PTH12,13
Subgroup analysis: Patients achieving iPTH < 600 pg/mL during EAP by screening iPTH3,12



Parsabiv® + vitamin D and/or phosphate binders†

Placebo + vitamin D and/or phosphate binders†
Overall, 76.5% of Parsabiv® patients (n = 497) achieved PTH < 600 pg/mL during the combined phase 3 trials.12
Secondary endpoint: In phase 3 trials, 53.4% of Parsabiv® patients achieved iPTH ≤ 300 pg/mL vs 5.8% of placebo patients during the EAP (P < 0.001).8
Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
- *KDIGO® guidelines suggest maintaining PTH in the range of 2x to 9x the upper limit of normal for the assay, defined as approximately 130 pg/mL to 600 pg/mL.13,14
- †Vitamin D and/or phosphate binders, if prescribed.3
- Note: All references to KDIGO® guidelines for CKD-MBD set forth herein are intended to be informational only and do not reflect KDIGO®’s endorsement or support of Parsabiv® and/or Amgen. KDIGO® is a registered trademark of the National Kidney Foundation, Inc.
- EAP = efficacy assessment phase; KDIGO® = Kidney Disease: Improving Global Outcomes.
What is Real-World Evidence and why does it matter?
from
REAL NEPHROLOGISTS
from
REAL PATIENTS
CONTROLLED
STUDY
Please review the placebo-controlled data, then the Real-World Evidence. Note that the Real-World Evidence is not derived from a controlled clinical study and no conclusions of statistical or clinical significance can be drawn.
Real-World Evidence collection criteria2
Retrospective analysis of patients collected from large and medium dialysis organizations, which traditionally have more protocol restrictions


Parsabiv® dosing was consistent with prescribing information
- First Parsabiv® prescription required 5 mg TIW dosing
- Baseline cCa ≥ 8.3 mg/dL for all patients
- *ALADIN is a 3rd-party national database of lab values of patients with CKD and sHPT being treated with hemodialysis.
Real-World Evidence:
64% of patients prescribed Parsabiv® achieved a > 30%
reduction PTH by week 242
Percentage of patients achieving > 30% reduction in mean iPTH from index date


Data are derived from real-world sources and not from a controlled clinical study. Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusion of statistical or clinical significance can be drawn.
Data represent patient lab outcomes provided to Amgen by large and mid-size dialysis organizations. All patients were initiated on calcimimetics between February 2018 and June 2020, had ≥ 90 days on treatment, with no observed gaps in therapy greater than 12 doses in 6 months or 24 doses in 9 months, no evidence of other calcimimetic therapy within 90 days of Parsabiv® therapy initiation, and no more than a 2-month gap in reported lab results for PTH. Patients with baseline PTH < 400 were excluded. All patients were initiated at Parsabiv® 5 mg TIW and had a baseline cCa ≥ 8.3 mg/dL.2
Real-World Evidence:By week 4, 37% of patients given Parsabiv® achieved a
> 30% reduction* in PTH2


date was first observed.
†Vitamin D and/or phosphate binders, if prescribed.2
Real-World Evidence:Parsabiv® achieved reductions in all 3 sHPT labs2


Real-World Evidence: Patients given Parsabiv® achieved PTH < 600 pg/mL at
week 24 when initiated at baseline PTH
≤ 1000 pg/mL2


Data are derived from real-world sources and not from a controlled clinical study. Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusion of statistical or clinical significance can be drawn.
Data represent patient lab outcomes provided to Amgen by large and mid-size dialysis organizations. All patients were initiated on calcimimetics between February 2018 and June 2020, had ≥ 90 days on treatment, with no observed gaps in therapy greater than 12 doses in 6 months or 24 doses in 9 months, no evidence of other calcimimetic therapy within 90 days of Parsabiv® therapy initiation, and no more than a 2-month gap in reported lab results for PTH. Patients with baseline PTH < 400 were excluded. All patients were initiated at Parsabiv® 5 mg TIW and had a baseline cCa ≥ 8.3 mg/dL.2
†KDIGO® guidelines suggest maintaining PTH in the range of 2x to 9x the upper limit of normal for the assay, defined as approximately 130 pg/mL to 600 pg/mL.4,5
Real-World Evidence:The majority of patients given Parsabiv® achieved the
KDIGO® goal range* for PTH2,4,6
% of patients who achieved iPTH < 600 pg/mL by week 242,6


Values represent percent of patients achieving the iPTH goal of < 600 pg/mL within 24 weeks. Overall, 77% of patients (n = 1535) achieved PTH < 600 pg/mL. 43% of patients (n= 859) achieved PTH ≤ 300 pg/mL.6
Data are derived from real-world sources and not from a controlled clinical study. Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusion of statistical or clinical significance can be drawn.
Data represent patient lab outcomes provided to Amgen by large and mid-size dialysis organizations. All patients were initiated on calcimimetics between February 2018 and June 2020, had ≥ 90 days on treatment, with no observed gaps in therapy greater than 12 doses in 6 months or 24 doses in 9 months, no evidence of other calcimimetic therapy within 90 days of Parsabiv® therapy initiation, and no more than a 2-month gap in reported lab results for PTH. Patients with baseline PTH < 400 were excluded. All patients were initiated at Parsabiv® 5 mg TIW and had a baseline cCa ≥ 8.3 mg/dL.2
Real-World Evidence:3 key lab levels and average Parsabiv® dose1,2,7,8


*Values represent mean iPTH during efficacy assessment phase (EAP), defined as weeks 20 through 27, inclusive.7
†Results are combined from two 26-week, randomized, double-blind, placebo-controlled studies comparing Parsabiv® with placebo in patients with CKD on hemodialysis.
Parsabiv® (etelcalcetide) was evaluated in a head-to-head study vs Sensipar® (oral cinacalcet) tablets4,5
Study Design


- A phase 3, 26-week, randomized, active-controlled, double-blind, double-dummy study comparing Parsabiv® with Sensipar® in patients with CKD on hemodialysis with iPTH > 500 pg/mL and corrected calcium ≥ 8.3 mg/dL; N = 683
- Parsabiv® IV TIW + daily oral placebo vs daily oral Sensipar® + placebo IV TIW for 26 weeks
- Mean baseline iPTH in the Parsabiv® group and Sensipar® group were 1092 pg/mL and 1139 pg/mL, respectively
- The average weekly dose of investigational product during the EAP (defined as weeks 20 through 27, inclusive) was 21 mg for Parsabiv® and 405 mg for Sensipar®
-
Adherence to investigational product through 26-week study period was:
- 97% for Parsabiv®
- 94% for Sensipar®
Titration4
- Parsabiv® (etelcalcetide) dose uptitrated from 5 mg in 2.5 mg or 5 mg increments, up to a maximum dose of 15 mg, at weeks 5, 9, 13, and 17 to target predialysis 100 ≤ iPTH ≤ 300 pg/mL while maintaining cCa ≥ 8.3 mg/dL
- Sensipar® dose uptitrated from 30 mg daily in 30 mg increments, up to a maximum dose of 180 mg daily, at weeks 5, 9, 13, and 17 to target predialysis 100 ≤ iPTH ≤ 300 pg/mL while maintaining cCa ≥ 8.3 mg/dL
- Parsabiv® was withheld if any of the following were observed: iPTH < 100 pg/mL (two consecutive measurements), corrected calcium < 7.5 mg/dL, symptomatic hypocalcemia, other drug-related adverse events
- Investigators were blinded to central laboratory serum iPTH values, and routine local iPTH monitoring during the study was suspended
Important Study Information
- At trial end, average Parsabiv® doses were higher than Sensipar® doses (relative to each product’s respective maximally recommended dose) and a greater proportion of Parsabiv®-treated subjects achieved a maximally recommended dose
-
The Parsabiv® arm had1:
- A higher relative starting dose
- Higher relative dose increments per dose escalation steps
- Fewer dose steps needed to reach the maximally recommended dose
- No differences in tolerability were identified to explain the lack of dose adjustment in the Sensipar® arm
Parsabiv® and Sensipar® patients who achieved a > 30% reduction from baseline in mean PTH4,5
Percentage of patients achieving > 30% reduction in mean iPTH from baseline


Parsabiv® (etelcalcetide) was non-inferior to Sensipar® with respect to the proportion of patients who achieved a > 30% reduction from baseline in mean iPTH during the EAP (77.9% vs 63.9%; estimated treatment difference of -10.5%; 95% CI: -17.45%, -3.51%); P < 0.001.4,5
Given the single, limited-duration (26-week) study design and important study information, these data should not be interpreted as providing evidence of superiority of Parsabiv® to Sensipar®. The potential impact of the difference between treatment groups on clinical outcomes has not been studied, and the clinical meaningfulness of such a difference is unknown.
Evaluating additional endpoints in the head-to-head study
Because the primary endpoint was met, the statistical analysis plan called for the sequential testing of the key secondary endpoints, including mean number of days of vomiting or nausea per week in the first 8 weeks.
- No statistically significant difference between the two groups was observed for the secondary endpoint evaluating the mean number of days of vomiting or nausea per week in the first 8 weeks
-
Therefore, other secondary and exploratory endpoints were subsequently evaluated, but were not formally tested for statistical significance. These endpoints included:
- Percent change from baseline in mean cCa during the EAP
- Percent change from baseline in mean phosphate during the EAP
- Percent change from baseline in iPTH during the EAP
In the head-to-head study, patients given Parsabiv® achieved reductions in all 3 key sHPT labs5
Mean iPTH, phosphate, and corrected calcium over time







Parsabiv® + vitamin D and/or phosphate binders*

Sensipar® (cinacalcet) + vitamin D and/or phosphate binders*
Mean % (absolute) change from baseline


Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
Given the single, limited-duration (26-week) study design and important study information, these data should not be interpreted as providing evidence of superiority of Parsabiv® (etelcalcetide) to Sensipar®. The potential impact of the difference between treatment groups on clinical outcomes has not been studied, and the clinical meaningfulness of such a difference is unknown.

CLINICAL REFERENCE GUIDE
Important clinical data to share with your colleagues

DOSING & MONITORING
Switching from oralcinacalcet to Parsabiv® (etelcalcetide)
REIMBURSEMENT
Parsabiv® reimbursement
options are available for
patients
IN REAL LIFE
Hear from your peers
about their experiences with Parsabiv®


